About us
RA-EWG Goal
In order to realize the APAC mission, RA-EWG will share information regarding the challenges faced in each country and transmit all necessary proposals to the stakeholders.
Furthermore, the pharmaceutical associations of each country will propose solutions to their respective governments and stakeholders regarding the pharmaceutical-related challenges in each region. All the activities to reach the goal are to be undertaken by the strategic approach including a step-by-step approach.
RA-EWG Leader’s Message
Areas of the RA-EWG focus are: 1) Implementing APAC Good Submission Practice (GSubP) as industry initiative. 2) Supporting promotion of Good Review Practice (GRevP) by making practical proposals from industry perspectives. 3) Promoting regulatory convergence of NDA requirements in Asia.
The RA-EWG has been advocating the concept of good registration management, under which execution of both Good Submission Practices (GSubP) and Good Review Practices (GRevP) by the applicants and the reviewers, respectively, will enhance the performance of both submission and review, leading to efficient and quick drug registration/approval.
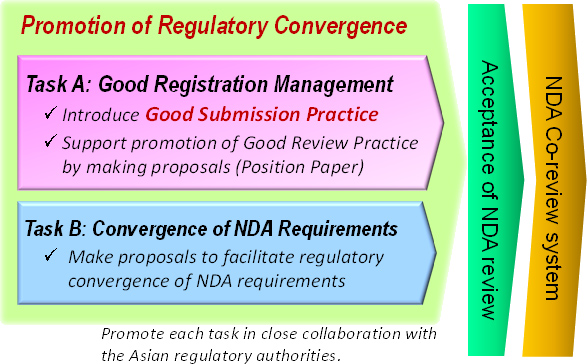
Goal and Approach of RA-EWG
Membership
Members are basically the IFPMA member associations in the following countries: